Peripheral T-Cell Non-Hodgkin Lymphoma Treatment (PDQ®): Treatment - Health Professional Information [NCI]
This information is produced and provided by the National Cancer Institute (NCI). The information in this topic may have changed since it was written. For the most current information, contact the National Cancer Institute via the Internet web site at http://cancer.gov or call 1-800-4-CANCER.
General Information About Peripheral T-Cell Non-Hodgkin Lymphoma
The non-Hodgkin lymphoma (NHL) T-cell lymphomas are a heterogeneous group of T-cell lymphoproliferative malignancies, which account for less than 15% of NHLs.[1] About 85% of NHL cases are B-cell lymphomas. For more information, see B-Cell Non-Hodgkin Lymphoma Treatment.
T-cell lymphoma can be divided into cutaneous T-cell lymphoma (CTCL), peripheral T-cell lymphoma (PTCL), and T-cell lymphoblastic lymphoma/acute lymphocytic leukemia (T-LBL/ALL).
T-LBL/ALL arises from very early T cells, often involves the thymus, and is more common in young adults. The lymphoma form is often treated similarly to the leukemia form. For more information, see Adult Acute Lymphoblastic Leukemia Treatment.
CTCL starts in the skin and includes mycosis fungoides, Sézary syndrome, primary cutaneous anaplastic large cell lymphoma, and others. For more information, see Mycosis Fungoides (Including Sézary Syndrome) Treatment.
PTCL originates from mature T cells. It usually arises from lymphoid tissues but can spread to other organs. Subsets of PTCL include anaplastic large cell lymphoma (ALCL), angioimmunoblastic T-cell lymphoma (AITL), extranodal natural killer/T-cell lymphoma (ENK/TCL), PTCL not otherwise specified (PTCL-NOS), adult T-cell leukemia/lymphoma (ATLL), enteropathy-associated T-cell lymphoma (EATL), hepatosplenic T-cell lymphoma (HSTCL), T-cell prolymphocytic leukemia (T-PLL), and others.
Incidence and Mortality
T-cell lymphomas make up less than 15% of NHL cases. Most T-cell lymphoma subtypes are associated with worse outcomes than those of B-cell lymphomas.[1]
Anatomy
NHL usually originates in lymphoid tissues.
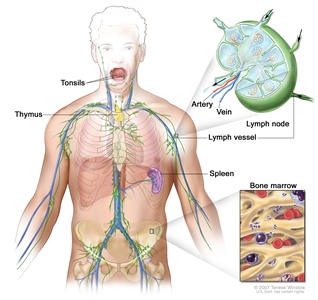
Anatomy of the lymph system.
Prognosis and Survival
Prognosis in PTCL varies depending on subtype, stage, and other factors. In general, PTCL is associated with a poor prognosis, with a 5-year survival rate of approximately 30% to 40%.[2,3] While outcomes are better for patients with ALK-positive ALCL, with a median 5-year overall survival (OS) closer to 70% to 80%,[2,3,4] other subsets are associated with worse survival, such as ALK-negative ALCL, AITL, PTCL-NOS, HSTCL, EATL, and ENK/TCL.[5,6]
Unlike B-cell NHLs, which include both indolent and aggressive forms, most PTCLs are considered aggressive.[7] As with most other aggressive lymphomas, PTCLs are often curable with systemic therapy, though effective treatment options are more limited, particularly in the relapsed or refractory setting.[8,9]
Even though existing treatments cure a significant fraction of patients with lymphoma, numerous clinical trials that explore treatment improvements are in progress. If possible, patients can be included in these studies.
In addition to screening for HIV among patients with aggressive lymphomas, active hepatitis B or hepatitis C can be assessed before treatment with chemotherapy.[10,11] Patients with detectable hepatitis B virus (HBV) benefit from prophylaxis with entecavir.[12,13] Patients with a resolved HBV infection (defined as hepatitis B surface antigen-negative but hepatitis B core antibody-positive) are at risk of reactivation of HBV and require monitoring of HBV DNA. Prophylactic nucleoside therapy lowered HBV reactivation from 10.8% to 2.1% in a retrospective study of 326 patients.[14] Prophylaxis for herpes zoster with acyclovir or valacyclovir and prophylaxis for pneumocystis with trimethoprim/sulfamethoxazole or dapsone are usually given to patients receiving combination chemotherapy.
References:
- American Cancer Society: Types of T-cell lymphoma. American Cancer Society, 2018. Available online. Last accessed June 16, 2023.
- Vose J, Armitage J, Weisenburger D, et al.: International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol 26 (25): 4124-30, 2008.
- Ellin F, Landström J, Jerkeman M, et al.: Real-world data on prognostic factors and treatment in peripheral T-cell lymphomas: a study from the Swedish Lymphoma Registry. Blood 124 (10): 1570-7, 2014.
- Sibon D, Fournier M, Brière J, et al.: Long-term outcome of adults with systemic anaplastic large-cell lymphoma treated within the Groupe d'Etude des Lymphomes de l'Adulte trials. J Clin Oncol 30 (32): 3939-46, 2012.
- Petrich AM, Helenowski IB, Bryan LJ, et al.: Factors predicting survival in peripheral T-cell lymphoma in the USA: a population-based analysis of 8802 patients in the modern era. Br J Haematol 168 (5): 708-18, 2015.
- Foss FM, Horwitz SM, Civallero M, et al.: Incidence and outcomes of rare T cell lymphomas from the T Cell Project: hepatosplenic, enteropathy associated and peripheral gamma delta T cell lymphomas. Am J Hematol 95 (2): 151-155, 2020.
- Armitage JO: The aggressive peripheral T-cell lymphomas: 2017. Am J Hematol 92 (7): 706-715, 2017.
- Bellei M, Foss FM, Shustov AR, et al.: The outcome of peripheral T-cell lymphoma patients failing first-line therapy: a report from the prospective, International T-Cell Project. Haematologica 103 (7): 1191-1197, 2018.
- Lansigan F, Horwitz SM, Pinter-Brown LC, et al.: Outcomes for Relapsed and Refractory Peripheral T-Cell Lymphoma Patients after Front-Line Therapy from the COMPLETE Registry. Acta Haematol 143 (1): 40-50, 2020.
- Niitsu N, Hagiwara Y, Tanae K, et al.: Prospective analysis of hepatitis B virus reactivation in patients with diffuse large B-cell lymphoma after rituximab combination chemotherapy. J Clin Oncol 28 (34): 5097-100, 2010.
- Dong HJ, Ni LN, Sheng GF, et al.: Risk of hepatitis B virus (HBV) reactivation in non-Hodgkin lymphoma patients receiving rituximab-chemotherapy: a meta-analysis. J Clin Virol 57 (3): 209-14, 2013.
- Huang YH, Hsiao LT, Hong YC, et al.: Randomized controlled trial of entecavir prophylaxis for rituximab-associated hepatitis B virus reactivation in patients with lymphoma and resolved hepatitis B. J Clin Oncol 31 (22): 2765-72, 2013.
- Li H, Zhang HM, Chen LF, et al.: Prophylactic lamivudine to improve the outcome of HBsAg-positive lymphoma patients during chemotherapy: a systematic review and meta-analysis. Clin Res Hepatol Gastroenterol 39 (1): 80-92, 2015.
- Kusumoto S, Arcaini L, Hong X, et al.: Risk of HBV reactivation in patients with B-cell lymphomas receiving obinutuzumab or rituximab immunochemotherapy. Blood 133 (2): 137-146, 2019.
Late Effects of Treatment of Peripheral T-Cell Non-Hodgkin Lymphoma
Late effects of treatment of non-Hodgkin lymphoma (NHL) have been observed. Impaired fertility may occur after exposure to alkylating agents.[1] For as many as three decades after diagnosis, patients are at a significantly elevated risk of developing second primary cancers, especially the following:[2,3,4,5]
- Lung cancer.
- Brain cancer.
- Kidney cancer.
- Bladder cancer.
- Melanoma.
- Hodgkin lymphoma.
- Acute nonlymphocytic leukemia.
Left ventricular dysfunction was a significant late effect in long-term survivors of high-grade NHL who received more than 200 mg/m² of doxorubicin.[1,6]
Myelodysplastic syndrome and acute myelogenous leukemia are late complications of myeloablative therapy with autologous bone marrow or peripheral blood stem cell support, as well as conventional chemotherapy-containing alkylating agents.[3,7,8,9,10,11,12,13,14] Most of these patients show clonal hematopoiesis even before the transplant, suggesting that the hematologic injury usually occurs during induction or reinduction chemotherapy.[9,15,16] A series of 605 patients who received autologous bone marrow transplant (BMT) with cyclophosphamide and total-body radiation therapy (as conditioning) were followed for a median of 10 years. The incidence of a second malignancy was 21%, and 10% of those malignancies were solid tumors.[17]
A study of young women who received autologous BMT reported successful pregnancies with children born free of congenital abnormalities.[18] Late-occurring venous thromboembolism can occur after allogeneic or autologous BMT.[19]
Some patients have osteopenia or osteoporosis at the start of therapy; bone density may worsen after therapy for lymphoma.[20]
References:
- Haddy TB, Adde MA, McCalla J, et al.: Late effects in long-term survivors of high-grade non-Hodgkin's lymphomas. J Clin Oncol 16 (6): 2070-9, 1998.
- Travis LB, Curtis RE, Glimelius B, et al.: Second cancers among long-term survivors of non-Hodgkin's lymphoma. J Natl Cancer Inst 85 (23): 1932-7, 1993.
- Mudie NY, Swerdlow AJ, Higgins CD, et al.: Risk of second malignancy after non-Hodgkin's lymphoma: a British Cohort Study. J Clin Oncol 24 (10): 1568-74, 2006.
- Hemminki K, Lenner P, Sundquist J, et al.: Risk of subsequent solid tumors after non-Hodgkin's lymphoma: effect of diagnostic age and time since diagnosis. J Clin Oncol 26 (11): 1850-7, 2008.
- Major A, Smith DE, Ghosh D, et al.: Risk and subtypes of secondary primary malignancies in diffuse large B-cell lymphoma survivors change over time based on stage at diagnosis. Cancer 126 (1): 189-201, 2020.
- Moser EC, Noordijk EM, van Leeuwen FE, et al.: Long-term risk of cardiovascular disease after treatment for aggressive non-Hodgkin lymphoma. Blood 107 (7): 2912-9, 2006.
- Darrington DL, Vose JM, Anderson JR, et al.: Incidence and characterization of secondary myelodysplastic syndrome and acute myelogenous leukemia following high-dose chemoradiotherapy and autologous stem-cell transplantation for lymphoid malignancies. J Clin Oncol 12 (12): 2527-34, 1994.
- Stone RM, Neuberg D, Soiffer R, et al.: Myelodysplastic syndrome as a late complication following autologous bone marrow transplantation for non-Hodgkin's lymphoma. J Clin Oncol 12 (12): 2535-42, 1994.
- Armitage JO, Carbone PP, Connors JM, et al.: Treatment-related myelodysplasia and acute leukemia in non-Hodgkin's lymphoma patients. J Clin Oncol 21 (5): 897-906, 2003.
- André M, Mounier N, Leleu X, et al.: Second cancers and late toxicities after treatment of aggressive non-Hodgkin lymphoma with the ACVBP regimen: a GELA cohort study on 2837 patients. Blood 103 (4): 1222-8, 2004.
- Oddou S, Vey N, Viens P, et al.: Second neoplasms following high-dose chemotherapy and autologous stem cell transplantation for malignant lymphomas: a report of six cases in a cohort of 171 patients from a single institution. Leuk Lymphoma 31 (1-2): 187-94, 1998.
- Lenz G, Dreyling M, Schiegnitz E, et al.: Moderate increase of secondary hematologic malignancies after myeloablative radiochemotherapy and autologous stem-cell transplantation in patients with indolent lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group. J Clin Oncol 22 (24): 4926-33, 2004.
- McLaughlin P, Estey E, Glassman A, et al.: Myelodysplasia and acute myeloid leukemia following therapy for indolent lymphoma with fludarabine, mitoxantrone, and dexamethasone (FND) plus rituximab and interferon alpha. Blood 105 (12): 4573-5, 2005.
- Morton LM, Curtis RE, Linet MS, et al.: Second malignancy risks after non-Hodgkin's lymphoma and chronic lymphocytic leukemia: differences by lymphoma subtype. J Clin Oncol 28 (33): 4935-44, 2010.
- Mach-Pascual S, Legare RD, Lu D, et al.: Predictive value of clonality assays in patients with non-Hodgkin's lymphoma undergoing autologous bone marrow transplant: a single institution study. Blood 91 (12): 4496-503, 1998.
- Lillington DM, Micallef IN, Carpenter E, et al.: Detection of chromosome abnormalities pre-high-dose treatment in patients developing therapy-related myelodysplasia and secondary acute myelogenous leukemia after treatment for non-Hodgkin's lymphoma. J Clin Oncol 19 (9): 2472-81, 2001.
- Brown JR, Yeckes H, Friedberg JW, et al.: Increasing incidence of late second malignancies after conditioning with cyclophosphamide and total-body irradiation and autologous bone marrow transplantation for non-Hodgkin's lymphoma. J Clin Oncol 23 (10): 2208-14, 2005.
- Jackson GH, Wood A, Taylor PR, et al.: Early high dose chemotherapy intensification with autologous bone marrow transplantation in lymphoma associated with retention of fertility and normal pregnancies in females. Scotland and Newcastle Lymphoma Group, UK. Leuk Lymphoma 28 (1-2): 127-32, 1997.
- Gangaraju R, Chen Y, Hageman L, et al.: Risk of venous thromboembolism in patients with non-Hodgkin lymphoma surviving blood or marrow transplantation. Cancer 125 (24): 4498-4508, 2019.
- Westin JR, Thompson MA, Cataldo VD, et al.: Zoledronic acid for prevention of bone loss in patients receiving primary therapy for lymphomas: a prospective, randomized controlled phase III trial. Clin Lymphoma Myeloma Leuk 13 (2): 99-105, 2013.
Cellular Classification of Peripheral T-Cell Non-Hodgkin Lymphoma
A pathologist should be consulted before a biopsy because some studies require special preparation of tissue (e.g., frozen tissue). Knowledge of cell surface markers and immunoglobulin and T-cell receptor gene rearrangements may help with diagnostic and therapeutic decisions. The clonal excess of light-chain immunoglobulin may differentiate malignant cells from reactive cells. Because the prognosis and the approach to treatment are influenced by histopathology, outside biopsy specimens should be carefully reviewed by a hematopathologist who is experienced in diagnosing lymphomas. Although lymph node biopsies are recommended whenever possible, sometimes immunophenotypic data are sufficient for diagnosis of lymphoma when fine-needle aspiration cytology or core needle biopsy is preferred.[1,2]
Current Classification Systems
Updated REAL/WHO classification
The World Health Organization (WHO) modification of the Revised European American Lymphoma (REAL) classification recognizes three major categories of lymphoid malignancies based on morphology and cell lineage: B-cell neoplasms, T-cell/natural killer (NK)-cell neoplasms, and Hodgkin lymphoma (HL). Both lymphomas and lymphoid leukemias are included in this classification because both solid and circulating phases are present in many lymphoid neoplasms and distinction between them is artificial. For example, B-cell chronic lymphocytic leukemia (CLL) and B-cell small lymphocytic lymphoma are simply different manifestations of the same neoplasm, as are lymphoblastic lymphomas and acute lymphocytic leukemias. Within the B-cell and T-cell categories, two subdivisions are recognized: precursor neoplasms, which correspond to the earliest stages of differentiation, and more mature differentiated neoplasms.[3,4]
B-cell neoplasms
- Precursor B-cell neoplasm: precursor B-acute lymphoblastic leukemia/lymphoblastic lymphoma (LBL).
- Peripheral B-cell neoplasms.
- B-cell CLL/small lymphocytic lymphoma.
- B-cell prolymphocytic leukemia.
- Lymphoplasmacytic lymphoma/immunocytoma.
- Mantle cell lymphoma.
- Follicular lymphoma.
- Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphatic tissue (MALT) type.
- Nodal marginal zone B-cell lymphoma (± monocytoid B cells).
- Splenic marginal zone lymphoma (± villous lymphocytes).
- Hairy cell leukemia.
- Plasmacytoma/plasma cell myeloma.
- Diffuse large B-cell lymphoma.
- Burkitt lymphoma.
T-cell and putative NK-cell neoplasms
- Precursor T-cell neoplasm: precursor T-acute lymphoblastic leukemia/LBL. For more information, see Adult Acute Lymphoblastic Leukemia Treatment.
- Peripheral T-cell and NK-cell neoplasms.
- T-cell CLL/prolymphocytic leukemia.
- T-cell granular lymphocytic leukemia.
- Mycosis fungoides (including Sézary syndrome).
- Peripheral T-cell lymphoma, not otherwise characterized.
- Hepatosplenic gamma/delta T-cell lymphoma.
- Subcutaneous panniculitis-like T-cell lymphoma.
- Angioimmunoblastic T-cell lymphoma.
- Extranodal T-/NK-cell lymphoma, nasal type.
- Enteropathy-type intestinal T-cell lymphoma.
- Adult T-cell lymphoma/leukemia (human T-lymphotrophic virus [HTLV] 1+).
- Anaplastic large cell lymphoma, primary systemic type.
- Anaplastic large cell lymphoma, primary cutaneous type.
- Aggressive NK-cell leukemia.
HL
- Nodular lymphocyte-predominant HL.
- Classical HL.
- Nodular sclerosis HL.
- Lymphocyte-rich classical HL.
- Mixed-cellularity HL.
- Lymphocyte-depleted HL.
The REAL classification encompasses all the lymphoproliferative neoplasms. For more information, see the following PDQ summaries:
- Adult Acute Lymphoblastic Leukemia Treatment
- Hodgkin Lymphoma Treatment
- AIDS-Related Lymphoma Treatment
- Chronic Lymphocytic Leukemia Treatment
- Hairy Cell Leukemia Treatment
- Mycosis Fungoides (Including Sézary Syndrome) Treatment
- Plasma Cell Neoplasms (Including Multiple Myeloma) Treatment
- Primary CNS Lymphoma Treatment
Subtypes of Peripheral T-Cell Non-Hodgkin Lymphoma
Peripheral T-cell non-Hodgkin lymphoma includes the following subtypes, among others:
- Anaplastic large cell lymphoma.
- Angioimmunoblastic T-cell lymphoma.
- Peripheral T-cell lymphoma, not otherwise specified.
- Extranodal NK/T-cell lymphoma.
- Enteropathy-type intestinal T-cell lymphoma.
- Hepatosplenic T-cell lymphoma.
- Adult T-cell leukemia/lymphoma.
- T-cell prolymphocytic leukemia. For more information, see Chronic Lymphocytic Leukemia Treatment.
- Cutaneous T-cell lymphoma (including mycosis fungoides and Sézary syndrome, subcutaneous panniculitis-like T-cell lymphoma, primary cutaneous anaplastic large cell lymphoma, primary cutaneous gamma-delta T-cell lymphoma, and others). For more information, see Mycosis Fungoides (Including Sézary Syndrome) Treatment.
- T-cell granular lymphocytic leukemia. For more information, see Chronic Lymphocytic Leukemia Treatment.
References:
- Zeppa P, Marino G, Troncone G, et al.: Fine-needle cytology and flow cytometry immunophenotyping and subclassification of non-Hodgkin lymphoma: a critical review of 307 cases with technical suggestions. Cancer 102 (1): 55-65, 2004.
- Young NA, Al-Saleem T: Diagnosis of lymphoma by fine-needle aspiration cytology using the revised European-American classification of lymphoid neoplasms. Cancer 87 (6): 325-45, 1999.
- Pileri SA, Milani M, Fraternali-Orcioni G, et al.: From the R.E.A.L. Classification to the upcoming WHO scheme: a step toward universal categorization of lymphoma entities? Ann Oncol 9 (6): 607-12, 1998.
- Society for Hematopathology Program: Society for Hematopathology Program. Am J Surg Pathol 21 (1): 114-121, 1997.
Stage Information for Peripheral T-Cell Non-Hodgkin Lymphoma
Stage is important in selecting a treatment for patients with non-Hodgkin lymphoma (NHL). Chest and abdominal computed tomography (CT) scans are usually part of the staging evaluation for all patients with lymphoma. The staging system for NHL is similar to the staging system used for Hodgkin lymphoma (HL).
It is common for patients with NHL to have involvement of the following sites:
- Noncontiguous lymph nodes.
- Waldeyer ring.
- Epitrochlear nodes.
- Gastrointestinal tract.
- Extranodal presentations. (A single extranodal site is occasionally the only site of involvement in patients with diffuse lymphoma.)
- Bone marrow.
- Liver (especially common in patients with low-grade lymphomas).
Cytological examination of cerebrospinal fluid may be positive in patients with aggressive NHL. Involvement of hilar and mediastinal lymph nodes is less common than in HL. Mediastinal adenopathy, however, is a prominent feature of lymphoblastic lymphoma and primary mediastinal B-cell lymphoma, entities primarily found in young adults.
Most patients with NHL present with advanced (stage III or stage IV) disease often identified by CT scans or biopsies of the bone marrow and other accessible sites of involvement. In a retrospective review of over 32,000 cases of lymphoma in France, up to 40% of diagnoses were made by core needle biopsy, and 60% were made by excisional biopsy.[1] After expert review, core needle biopsy provided a definite diagnosis in 92.3% of cases; excisional biopsy provided a definite diagnosis in 98.1% of cases (P < .0001). Laparoscopic biopsy or laparotomy is not required for staging but rarely may be necessary to establish a diagnosis or histological type.[2]
Positron emission tomography (PET) with fluorine F 18-fludeoxyglucose can be used for initial staging. It can also be used for follow-up after therapy as a supplement to CT scanning.[3] Multiple studies have demonstrated that interim PET scans after two to four cycles of therapy do not provide reliable prognostic information. A large cooperative group trial (ECOG-E344 [NCT00274924]) reported problems with interobserver reproducibility. Two prospective trials and one meta-analysis showed no differences in outcomes between PET-negative and PET-positive/biopsy-negative patients.[4,5,6,7]
In a retrospective study of 130 patients with diffuse large B-cell lymphoma, PET scanning identified all clinically important marrow involvement from lymphoma, and bone marrow biopsy did not upstage any patient's lymphoma.[8] A retrospective study of 580 patients with follicular lymphoma from seven National Cancer Institute–sponsored trials showed no improvement in assessing response to therapy when bone marrow biopsy was added to radiological imaging.[9] The workup of NHL should include bone marrow biopsy when management would change (e.g., determining limited stage vs. advanced stage) or when evaluating cytopenias.
Staging Subclassification System
Lugano classification
The American Joint Committee on Cancer (AJCC) has adopted the Lugano classification to evaluate and stage lymphoma.[10] The Lugano classification system replaces the Ann Arbor classification system, which was adopted in 1971 at the Ann Arbor Conference,[11] with some modifications 18 years later from the Cotswolds meeting.[12,13]
| Stage | Stage Description | Illustration |
|---|---|---|
| CSF = cerebrospinal fluid; CT = computed tomography; DLBCL = diffuse large B-cell lymphoma; NHL = non-Hodgkin lymphoma. | ||
| a Hodgkin and Non-Hodgkin Lymphomas. In: Amin MB, Edge SB, Greene FL, et al., eds.:AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 937–58. | ||
| b Stage II bulky may be considered either early or advanced stage based on lymphoma histology and prognostic factors. | ||
| c The definition of disease bulk varies according to lymphoma histology. In the Lugano classification, bulk ln Hodgkin lymphoma is defined as a mass greater than one-third of the thoracic diameter on CT of the chest or a mass >10 cm. For NHL, the recommended definitions of bulk vary by lymphoma histology. In follicular lymphoma, 6 cm has been suggested based on the Follicular Lymphoma International Prognostic Index-2 and its validation. In DLBCL, cutoffs ranging from 5 cm to 10 cm have been used, although 10 cm is recommended. | ||
| Limited stage | ||
| I | Involvement of a single lymphatic site (i.e., nodal region, Waldeyer's ring, thymus, or spleen). | 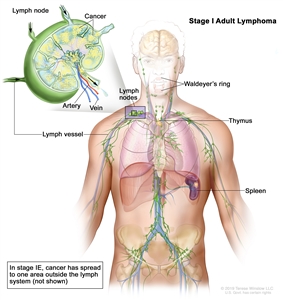 |
| IE | Single extralymphatic site in the absence of nodal involvement (rare in Hodgkin lymphoma). | |
| II | Involvement of two or more lymph node regions on the same side of the diaphragm. | 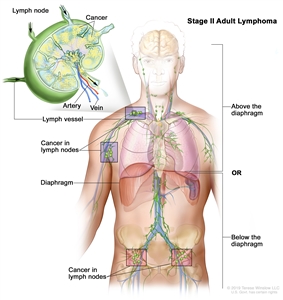 |
| IIE | Contiguous extralymphatic extension from a nodal site with or without involvement of other lymph node regions on the same side of the diaphragm. | 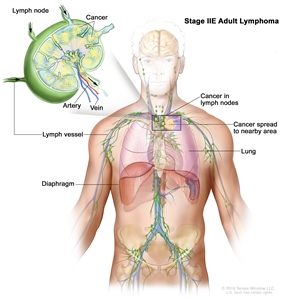 |
| II bulkyb | Stage II with disease bulk.c | |
| Advanced stage | ||
| III | Involvement of lymph node regions on both sides of the diaphragm; nodes above the diaphragm with spleen involvement. | 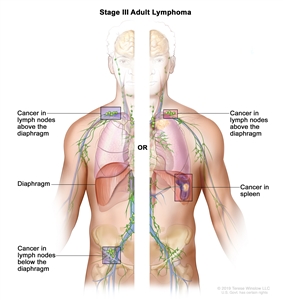 |
| IV | Diffuse or disseminated involvement of one or more extralymphatic organs, with or without associated lymph node involvement; or noncontiguous extralymphatic organ involvement in conjunction with nodal stage II disease; or any extralymphatic organ involvement in nodal stage III disease. Stage IV includes any involvement of the CSF, bone marrow, liver, or multiple lung lesions (other than by direct extension in stage IIE disease). | 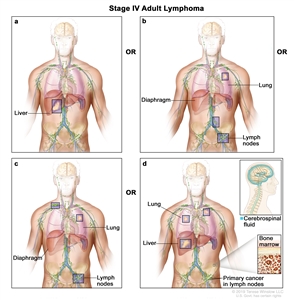 |
| Note: Hodgkin lymphoma uses A or B designation with stage group. A/B is no longer used in NHL. | ||
Occasionally, specialized staging systems are used. The physician should be aware of the system used in a specific report.
The E designation is used when extranodal lymphoid malignancies arise in tissues separate from, but near, the major lymphatic aggregates. Stage IV refers to disease that is diffusely spread throughout an extranodal site, such as the liver. If pathological proof of involvement of one or more extralymphatic sites has been documented, the symbol for the site of involvement, followed by a plus sign (+), is listed.
| N = nodes | H = liver | L = lung | M = bone marrow |
| S = spleen | P = pleura | O = bone | D = skin |
Current practice assigns a clinical stage based on the findings of the clinical evaluation and a pathological stage based on the findings from invasive procedures beyond the initial biopsy.
For example, on percutaneous biopsy, a patient with inguinal adenopathy and a positive lymphangiogram without systemic symptoms might have involvement of the liver and bone marrow. The precise stage of such a patient would be clinical stage IIA, pathological stage IVA(H+)(M+).
Several other factors that are not included in the above staging system are important for the staging and prognosis of patients with NHL. These factors include the following:
- Age.
- Performance status (PS).
- Tumor size.
- Lactate dehydrogenase (LDH) values.
- The number of extranodal sites.
The National Comprehensive Cancer Network International Prognostic Index (IPI) for aggressive NHL (diffuse large cell lymphoma) identifies the following five significant risk factors prognostic of overall survival (OS) and their associated risk scores:[14]
- Age.
- <40 years: 0.
- 41–60 years: 1.
- 61–75 years: 2.
- >75 years: 3.
- Stage III/IV: 1.
- Performance status (PS) 2/3/4: 1.
- Serum lactate dehydrogenase (LDH).
- Normalized: 0.
- >1x–3x: 1.
- >3x: 2.
- Number of extranodal sites ≥2: 1.
Risk scores:
- Low (0 or 1): 5-year OS rate, 96%; progression-free survival (PFS) rate, 91%.
- Low intermediate (2 or 3): 5-year OS rate, 82%; PFS rate, 74%.
- High intermediate (4 or 5): 5-year OS rate, 64%; PFS rate, 51%.
- High (>6): 5-year OS rate, 33%; PFS rate, 30%.
References:
- Syrykh C, Chaouat C, Poullot E, et al.: Lymph node excisions provide more precise lymphoma diagnoses than core biopsies: a French Lymphopath network survey. Blood 140 (24): 2573-2583, 2022.
- Mann GB, Conlon KC, LaQuaglia M, et al.: Emerging role of laparoscopy in the diagnosis of lymphoma. J Clin Oncol 16 (5): 1909-15, 1998.
- Barrington SF, Mikhaeel NG, Kostakoglu L, et al.: Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol 32 (27): 3048-58, 2014.
- Horning SJ, Juweid ME, Schöder H, et al.: Interim positron emission tomography scans in diffuse large B-cell lymphoma: an independent expert nuclear medicine evaluation of the Eastern Cooperative Oncology Group E3404 study. Blood 115 (4): 775-7; quiz 918, 2010.
- Moskowitz CH, Schöder H, Teruya-Feldstein J, et al.: Risk-adapted dose-dense immunochemotherapy determined by interim FDG-PET in Advanced-stage diffuse large B-Cell lymphoma. J Clin Oncol 28 (11): 1896-903, 2010.
- Pregno P, Chiappella A, Bellò M, et al.: Interim 18-FDG-PET/CT failed to predict the outcome in diffuse large B-cell lymphoma patients treated at the diagnosis with rituximab-CHOP. Blood 119 (9): 2066-73, 2012.
- Sun N, Zhao J, Qiao W, et al.: Predictive value of interim PET/CT in DLBCL treated with R-CHOP: meta-analysis. Biomed Res Int 2015: 648572, 2015.
- Khan AB, Barrington SF, Mikhaeel NG, et al.: PET-CT staging of DLBCL accurately identifies and provides new insight into the clinical significance of bone marrow involvement. Blood 122 (1): 61-7, 2013.
- Rutherford SC, Yin J, Pederson L, et al.: Relevance of Bone Marrow Biopsies for Response Assessment in US National Cancer Institute National Clinical Trials Network Follicular Lymphoma Clinical Trials. J Clin Oncol 41 (2): 336-342, 2023.
- Hodgkin and non-Hodgkin lymphoma. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp. 937–58.
- Carbone PP, Kaplan HS, Musshoff K, et al.: Report of the Committee on Hodgkin's Disease Staging Classification. Cancer Res 31 (11): 1860-1, 1971.
- Lister TA, Crowther D, Sutcliffe SB, et al.: Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin's disease: Cotswolds meeting. J Clin Oncol 7 (11): 1630-6, 1989.
- National Cancer Institute sponsored study of classifications of non-Hodgkin's lymphomas: summary and description of a working formulation for clinical usage. The Non-Hodgkin's Lymphoma Pathologic Classification Project. Cancer 49 (10): 2112-35, 1982.
- Zhou Z, Sehn LH, Rademaker AW, et al.: An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood 123 (6): 837-42, 2014.
Treatment of Anaplastic Large Cell Lymphoma
Anaplastic large cell lymphoma (ALCL) is a peripheral T-cell lymphoma associated with the CD30 antigen. The translocation of chromosomes 2 and 5 creates a unique fusion protein with a nucleophosmin–anaplastic lymphoma kinase (ALK).[1,2] Patients whose lymphomas express ALK by immunohistochemistry are usually younger and may have systemic symptoms, extranodal disease, and advanced-stage disease. However, they have a more favorable survival rate than patients with ALK-negative disease.[3,4]
- A prospective randomized trial included 452 patients with CD30-positive T-cell lymphoma (CD30 expression >10%). Of these patients, 70% had ALCL (22% with ALK-positive disease and 48% with ALK-negative disease). The trial compared the previously used standard regimen, CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), with brentuximab vedotin (an anti-CD30 monoclonal antibody conjugated to a cytotoxic agent) combined with cyclophosphamide, doxorubicin, and prednisone (A+CHP regimen).[5]
- With a median follow-up of 47.6 months, the 5-year overall survival (OS) rates were 70.1% (95% confidence interval [CI], 63.3%–75.9%) for patients who received A+CHP and 61.0% (95% CI, 54.0%–67.3%) for patients who received CHOP (hazard ratio [HR], 0.72; 95% CI, 0.53–0.99).[6][Level of evidence A1]
- The 5-year progression-free survival (PFS) rates were 51.4% (95% CI, 42.8%–59.4%) for patients who received A+CHP and 43.0% (95% CI, 35.8%–50.0%) for patients who received CHOP (HR, 0.70; 95% CI, 0.53–0.91).
- This established A+CHP as a new option for patients with ALCL or other CD30-positive T-cell lymphomas, such as angioimmunoblastic T-cell lymphoma and peripheral T-cell lymphoma, not otherwise specified.
- For patients with relapsed disease, anecdotal responses have been reported for brentuximab vedotin,[7,8,9,10] romidepsin,[11] and pralatrexate.[12][Level of evidence C3]
- In a phase II study (NCT00866047), 66% of 58 patients attained a complete response with brentuximab vedotin.[10]
- At a median follow-up of 58 months, the 5-year PFS rate was 57% (95% CI, 41%–74%), and the 5-year OS rate was 79% (95% CI, 65%–92%). Of the patients achieving a complete response, 42% underwent hematopoietic stem cell transplant (SCT).[10][Level of evidence C3]
- In a retrospective review, 39 patients with relapsed disease had a 3-year PFS rate of 50% after autologous or allogeneic SCT.[13][Level of evidence C2]
- A retrospective review of 84 patients with ALK-negative ALCL suggested a survival benefit with autologous SCT. This hypothesis requires confirmation in a randomized prospective trial.[14][Level of evidence C3]
- A retrospective study included 182 patients with relapsed or refractory ALCL (23% ALK-positive, 21% ALK-negative, and 56% ALK-unknown) who underwent allogeneic SCT.[15]
- The 5-year PFS rate was 41% (95% CI, 34%–49%), and the 5-year OS rate was 41% (95% CI, 49%–64%).[15][Level of evidence C3]
- On multivariate analysis, African American race (HR, 2.7; 95% CI, 1.6–4.8; P < .001) and refractory disease at time of allogeneic SCT (HR, 3.2; 95% CI, 1.6–6.2; P < .001) were predictive factors for inferior OS.
- Despite ALK positivity being a favorable prognostic factor, outcomes after allogeneic SCT in this study did not vary significantly according to ALK status.
ALCL in children is usually characterized by systemic and cutaneous disease and has high response rates and good OS with doxorubicin-based combination chemotherapy.[16] The ALK inhibitor crizotinib has been combined with chemotherapy for previously untreated pediatric patients, and crizotinib has been used to control disease in multiply relapsed pediatric patients.[17,18] Crizotinib is associated with a high risk (around 25%) of thromboembolism, especially pulmonary embolism, and prophylaxis is recommended. There are no reports supporting the use of crizotinib in adults.
Patients with breast implant–associated ALCL may do well without chemotherapy after capsulectomy and implant removal if the disease is confined to the fibrous capsule, and no associated mass or lymphadenopathy is present.[19,20,21,22] Most patients with breast implant–associated ALCL have a characteristic deletion at 20Q13.13 that may help to diagnostically distinguish it from cutaneous or systemic ALCL.[23]
Primary cutaneous ALCL is a distinct entity that is typically ALK-negative and has a very indolent/low-grade clinical course.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References:
- Bai RY, Ouyang T, Miething C, et al.: Nucleophosmin-anaplastic lymphoma kinase associated with anaplastic large-cell lymphoma activates the phosphatidylinositol 3-kinase/Akt antiapoptotic signaling pathway. Blood 96 (13): 4319-27, 2000.
- Hapgood G, Savage KJ: The biology and management of systemic anaplastic large cell lymphoma. Blood 126 (1): 17-25, 2015.
- Gascoyne RD, Aoun P, Wu D, et al.: Prognostic significance of anaplastic lymphoma kinase (ALK) protein expression in adults with anaplastic large cell lymphoma. Blood 93 (11): 3913-21, 1999.
- Sibon D, Fournier M, Brière J, et al.: Long-term outcome of adults with systemic anaplastic large-cell lymphoma treated within the Groupe d'Etude des Lymphomes de l'Adulte trials. J Clin Oncol 30 (32): 3939-46, 2012.
- Horwitz S, O'Connor OA, Pro B, et al.: Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet 393 (10168): 229-240, 2019.
- Horwitz S, O'Connor OA, Pro B, et al.: The ECHELON-2 Trial: 5-year results of a randomized, phase III study of brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma. Ann Oncol 33 (3): 288-298, 2022.
- Younes A, Bartlett NL, Leonard JP, et al.: Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med 363 (19): 1812-21, 2010.
- Pro B, Advani R, Brice P, et al.: Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol 30 (18): 2190-6, 2012.
- Prince HM, Kim YH, Horwitz SM, et al.: Brentuximab vedotin or physician's choice in CD30-positive cutaneous T-cell lymphoma (ALCANZA): an international, open-label, randomised, phase 3, multicentre trial. Lancet 390 (10094): 555-566, 2017.
- Pro B, Advani R, Brice P, et al.: Five-year results of brentuximab vedotin in patients with relapsed or refractory systemic anaplastic large cell lymphoma. Blood 130 (25): 2709-2717, 2017.
- Coiffier B, Pro B, Prince HM, et al.: Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J Clin Oncol 30 (6): 631-6, 2012.
- O'Connor OA, Horwitz S, Hamlin P, et al.: Phase II-I-II study of two different doses and schedules of pralatrexate, a high-affinity substrate for the reduced folate carrier, in patients with relapsed or refractory lymphoma reveals marked activity in T-cell malignancies. J Clin Oncol 27 (26): 4357-64, 2009.
- Smith SM, Burns LJ, van Besien K, et al.: Hematopoietic cell transplantation for systemic mature T-cell non-Hodgkin lymphoma. J Clin Oncol 31 (25): 3100-9, 2013.
- Brink M, Meeuwes FO, van der Poel MWM, et al.: Impact of etoposide and ASCT on survival among patients aged <65 years with stage II to IV PTCL: a population-based cohort study. Blood 140 (9): 1009-1019, 2022.
- Furqan F, Ahn KW, Chen Y, et al.: Allogeneic haematopoietic cell transplant in patients with relapsed/refractory anaplastic large cell lymphoma. Br J Haematol 200 (1): 54-63, 2023.
- Seidemann K, Tiemann M, Schrappe M, et al.: Short-pulse B-non-Hodgkin lymphoma-type chemotherapy is efficacious treatment for pediatric anaplastic large cell lymphoma: a report of the Berlin-Frankfurt-Münster Group Trial NHL-BFM 90. Blood 97 (12): 3699-706, 2001.
- Lowe EJ, Reilly AF, Lim MS, et al.: Crizotinib in Combination With Chemotherapy for Pediatric Patients With ALK+ Anaplastic Large-Cell Lymphoma: The Results of Children's Oncology Group Trial ANHL12P1. J Clin Oncol 41 (11): 2043-2053, 2023.
- Mossé YP, Voss SD, Lim MS, et al.: Targeting ALK With Crizotinib in Pediatric Anaplastic Large Cell Lymphoma and Inflammatory Myofibroblastic Tumor: A Children's Oncology Group Study. J Clin Oncol 35 (28): 3215-3221, 2017.
- Miranda RN, Aladily TN, Prince HM, et al.: Breast implant-associated anaplastic large-cell lymphoma: long-term follow-up of 60 patients. J Clin Oncol 32 (2): 114-20, 2014.
- Clemens MW, Medeiros LJ, Butler CE, et al.: Complete Surgical Excision Is Essential for the Management of Patients With Breast Implant-Associated Anaplastic Large-Cell Lymphoma. J Clin Oncol 34 (2): 160-8, 2016.
- Mehta-Shah N, Clemens MW, Horwitz SM: How I treat breast implant-associated anaplastic large cell lymphoma. Blood 132 (18): 1889-1898, 2018.
- Jaffe ES, Ashar BS, Clemens MW, et al.: Best Practices Guideline for the Pathologic Diagnosis of Breast Implant-Associated Anaplastic Large-Cell Lymphoma. J Clin Oncol 38 (10): 1102-1111, 2020.
- Los-de Vries GT, de Boer M, van Dijk E, et al.: Chromosome 20 loss is characteristic of breast implant-associated anaplastic large cell lymphoma. Blood 136 (25): 2927-2932, 2020.
Treatment of Angioimmunoblastic T-Cell Lymphoma
Angioimmunoblastic T-cell lymphoma (AITL or ATCL) was formerly called angioimmunoblastic lymphadenopathy with dysproteinemia. Characterized by clonal T-cell receptor gene rearrangement, this entity is treated like diffuse large B-cell lymphoma, using cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) (without rituximab).[1,2,3,4] Patients present with profound lymphadenopathy, fever, night sweats, weight loss, skin rash, a positive Coombs test, and polyclonal hypergammaglobulinemia.[5] Opportunistic infections are frequent because of an underlying immune deficiency. B-cell Epstein-Barr virus genomes are detected in most affected patients.[6] For more information about weight loss, see Nutrition in Cancer Care and for more information about skin rash, see Pruritus.
Doxorubicin-based combination chemotherapy, such as the CHOP regimen, is commonly used for AITL, as it is for other aggressive lymphomas.[1,4] For CD30-positive cases, brentuximab vedotin combined with cyclophosphamide, doxorubicin, and prednisone is the proposed standard of care.[7][Level of evidence C3] Patients with AITL were included in the clinical trial involving mostly patients with anaplastic large cell lymphoma; a benefit for this smaller AITL subgroup cannot be established.[7,8][Level of evidence C3] For more information, see the Treatment of Anaplastic Large Cell Lymphoma section.
The International Peripheral T-Cell Lymphoma Project involving 22 international centers identified 243 patients with AITL or ATCL; the 5-year overall survival rate was 33% and the failure-free survival rate was 18%.[9] Myeloablative chemotherapy and radiation therapy with autologous or allogeneic peripheral stem cell support has been described in anecdotal reports.[10,11,12,13,14,15][Level of evidence C3] Anecdotal responses have been reported for cyclosporine,[16] pralatrexate,[17] bendamustine,[18] the histone deacetylase inhibitor romidepsin, and brentuximab vedotin (even if there is little or no CD30 expression on the lymphoma).[19,20][Level of evidence C3] Occasional spontaneous remissions and protracted responses to steroids only have been reported.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References:
- Siegert W, Agthe A, Griesser H, et al.: Treatment of angioimmunoblastic lymphadenopathy (AILD)-type T-cell lymphoma using prednisone with or without the COPBLAM/IMVP-16 regimen. A multicenter study. Kiel Lymphoma Study Group. Ann Intern Med 117 (5): 364-70, 1992.
- Jaffe ES: Angioimmunoblastic T-cell lymphoma: new insights, but the clinical challenge remains. Ann Oncol 6 (7): 631-2, 1995.
- Siegert W, Nerl C, Agthe A, et al.: Angioimmunoblastic lymphadenopathy (AILD)-type T-cell lymphoma: prognostic impact of clinical observations and laboratory findings at presentation. The Kiel Lymphoma Study Group. Ann Oncol 6 (7): 659-64, 1995.
- Lunning MA, Vose JM: Angioimmunoblastic T-cell lymphoma: the many-faced lymphoma. Blood 129 (9): 1095-1102, 2017.
- Rizvi MA, Evens AM, Tallman MS, et al.: T-cell non-Hodgkin lymphoma. Blood 107 (4): 1255-64, 2006.
- Bräuninger A, Spieker T, Willenbrock K, et al.: Survival and clonal expansion of mutating "forbidden" (immunoglobulin receptor-deficient) epstein-barr virus-infected b cells in angioimmunoblastic t cell lymphoma. J Exp Med 194 (7): 927-40, 2001.
- Horwitz S, O'Connor OA, Pro B, et al.: Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet 393 (10168): 229-240, 2019.
- Horwitz S, O'Connor OA, Pro B, et al.: The ECHELON-2 Trial: 5-year results of a randomized, phase III study of brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma. Ann Oncol 33 (3): 288-298, 2022.
- Federico M, Rudiger T, Bellei M, et al.: Clinicopathologic characteristics of angioimmunoblastic T-cell lymphoma: analysis of the international peripheral T-cell lymphoma project. J Clin Oncol 31 (2): 240-6, 2013.
- Reimer P, Rüdiger T, Geissinger E, et al.: Autologous stem-cell transplantation as first-line therapy in peripheral T-cell lymphomas: results of a prospective multicenter study. J Clin Oncol 27 (1): 106-13, 2009.
- Le Gouill S, Milpied N, Buzyn A, et al.: Graft-versus-lymphoma effect for aggressive T-cell lymphomas in adults: a study by the Société Francaise de Greffe de Moëlle et de Thérapie Cellulaire. J Clin Oncol 26 (14): 2264-71, 2008.
- Kyriakou C, Canals C, Finke J, et al.: Allogeneic stem cell transplantation is able to induce long-term remissions in angioimmunoblastic T-cell lymphoma: a retrospective study from the lymphoma working party of the European group for blood and marrow transplantation. J Clin Oncol 27 (24): 3951-8, 2009.
- Park SI, Horwitz SM, Foss FM, et al.: The role of autologous stem cell transplantation in patients with nodal peripheral T-cell lymphomas in first complete remission: Report from COMPLETE, a prospective, multicenter cohort study. Cancer 125 (9): 1507-1517, 2019.
- Brink M, Meeuwes FO, van der Poel MWM, et al.: Impact of etoposide and ASCT on survival among patients aged <65 years with stage II to IV PTCL: a population-based cohort study. Blood 140 (9): 1009-1019, 2022.
- Los-de Vries GT, de Boer M, van Dijk E, et al.: Chromosome 20 loss is characteristic of breast implant-associated anaplastic large cell lymphoma. Blood 136 (25): 2927-2932, 2020.
- Advani R, Horwitz S, Zelenetz A, et al.: Angioimmunoblastic T cell lymphoma: treatment experience with cyclosporine. Leuk Lymphoma 48 (3): 521-5, 2007.
- Amengual JE, Lichtenstein R, Lue J, et al.: A phase 1 study of romidepsin and pralatrexate reveals marked activity in relapsed and refractory T-cell lymphoma. Blood 131 (4): 397-407, 2018.
- Damaj G, Gressin R, Bouabdallah K, et al.: Results from a prospective, open-label, phase II trial of bendamustine in refractory or relapsed T-cell lymphomas: the BENTLY trial. J Clin Oncol 31 (1): 104-10, 2013.
- Coiffier B, Pro B, Prince HM, et al.: Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J Clin Oncol 30 (6): 631-6, 2012.
- Fanale MA, Horwitz SM, Forero-Torres A, et al.: Five-year outcomes for frontline brentuximab vedotin with CHP for CD30-expressing peripheral T-cell lymphomas. Blood 131 (19): 2120-2124, 2018.
Treatment of Peripheral T-Cell Lymphoma, Not Otherwise Specified
Patients with peripheral T-cell lymphoma, not otherwise specified (PTCL-NOS) have diffuse large cell or diffuse mixed lymphoma that expresses a cell surface phenotype of a postthymic (or peripheral) T-cell expressing CD4 or CD8 but not both together.[1] PTCL-NOS encompasses a group of heterogeneous nodal T-cell lymphomas that will require future delineation.[2,3]
Prognosis
Most investigators report worse response and survival rates for patients with PTCL-NOS than for patients with comparably staged B-cell aggressive lymphomas.[3,4] Most patients present with multiple adverse prognostic factors (i.e., older age, stage IV, multiple extranodal sites, and elevated lactate dehydrogenase), and these patients have low (<20%) failure-free survival and overall survival (OS) rates at 5 years.[3,4] As with other lymphomas (e.g., diffuse large B-cell lymphoma [DLBCL] or follicular lymphoma), event-free survival (EFS) at 24 months predicts a 5-year OS rate of 78%.[5]
Therapeutic Approaches
Therapy involves doxorubicin-based combination chemotherapy such as cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or CHOP plus etoposide (CHOPE). Doses are the same as those used for DLBCL.[6] For CD30-positive cases, brentuximab vedotin combined with cyclophosphamide, doxorubicin, and prednisone is the proposed standard of care.[7][Level of evidence C3] Patients with PTCL-NOS were included in the clinical trial involving mostly patients with anaplastic large cell lymphoma; a benefit for this smaller PTCL-NOS subgroup cannot be established.[7,8][Level of evidence C3] For more information, see the Treatment of Anaplastic Large Cell Lymphoma section.
For patients with early-stage disease, anecdotal retrospective series disagree on the value of consolidative radiation therapy after combination chemotherapy.[9][Level of evidence C3] Consolidation therapy using high-dose chemotherapy with autologous or allogeneic hematopoietic stem cell transplant (SCT) has been given to patients with advanced-stage PTCL after induction therapy in multiple phase II or retrospective trials. Evidence for this approach is anecdotal.[10,11,12,13,14,15,16,17][Level of evidence C3]
- A randomized prospective trial included 104 patients younger than 61 years with stage II, III, or IV PTCL (excluding ALK-positive anaplastic large cell lymphoma). Patients received either autologous SCT or allogeneic SCT as consolidation therapy after induction with CHOPE followed by DHAP (dexamethasone, cytarabine, and cisplatin).[18][Level of evidence C3]
- With a median follow-up of 42 months, the 3-year EFS rate was 43% for patients who received allogeneic SCT and 38% for patients who received autologous SCT.
- The 3-year OS rate was 57% for patients who received allogeneic SCT and 70% for patients who received autologous SCT (P = nonsignificant).
- None of the 21 responding patients who proceeded to allogeneic SCT relapsed, and 36% of patients who proceeded to autologous SCT relapsed.
- Eight of 26 patients (31%) who received allogeneic SCT died of graft-versus-host disease, and none of the 41 patients who received autologous SCT died of toxicity.
- The benefit of graft-versus-lymphoma effect was negated by increased transplant-related mortality.
- In a prospective trial of 109 evaluable patients with relapsing disease, treatment with pralatrexate resulted in a 30% response rate and a median 10-month duration of response.[19,20][Level of evidence C3]
- Similar response rates were seen in 130 evaluable patients with relapsing disease who received romidepsin in a prospective trial.[20][Level of evidence C3]
- Anecdotal responses have been seen with a combination of pralatrexate and romidepsin,[21] single-agent bendamustine,[22] belinostat,[23] and brentuximab vedotin (even if there is little or no CD30 expression on the lymphoma).[24][Level of evidence C3]
Incorporation of these new agents with CHOP chemotherapy is under clinical evaluation.[3,7]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References:
- Rüdiger T, Weisenburger DD, Anderson JR, et al.: Peripheral T-cell lymphoma (excluding anaplastic large-cell lymphoma): results from the Non-Hodgkin's Lymphoma Classification Project. Ann Oncol 13 (1): 140-9, 2002.
- Rizvi MA, Evens AM, Tallman MS, et al.: T-cell non-Hodgkin lymphoma. Blood 107 (4): 1255-64, 2006.
- Weisenburger DD, Savage KJ, Harris NL, et al.: Peripheral T-cell lymphoma, not otherwise specified: a report of 340 cases from the International Peripheral T-cell Lymphoma Project. Blood 117 (12): 3402-8, 2011.
- Sonnen R, Schmidt WP, Müller-Hermelink HK, et al.: The International Prognostic Index determines the outcome of patients with nodal mature T-cell lymphomas. Br J Haematol 129 (3): 366-72, 2005.
- Maurer MJ, Ellin F, Srour L, et al.: International Assessment of Event-Free Survival at 24 Months and Subsequent Survival in Peripheral T-Cell Lymphoma. J Clin Oncol 35 (36): 4019-4026, 2017.
- Carson KR, Horwitz SM, Pinter-Brown LC, et al.: A prospective cohort study of patients with peripheral T-cell lymphoma in the United States. Cancer 123 (7): 1174-1183, 2017.
- Horwitz S, O'Connor OA, Pro B, et al.: Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet 393 (10168): 229-240, 2019.
- Horwitz S, O'Connor OA, Pro B, et al.: The ECHELON-2 Trial: 5-year results of a randomized, phase III study of brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma. Ann Oncol 33 (3): 288-298, 2022.
- Briski R, Feldman AL, Bailey NG, et al.: Survival in patients with limited-stage peripheral T-cell lymphomas. Leuk Lymphoma 56 (6): 1665-70, 2015.
- Rodriguez J, Munsell M, Yazji S, et al.: Impact of high-dose chemotherapy on peripheral T-cell lymphomas. J Clin Oncol 19 (17): 3766-70, 2001.
- Reimer P, Rüdiger T, Geissinger E, et al.: Autologous stem-cell transplantation as first-line therapy in peripheral T-cell lymphomas: results of a prospective multicenter study. J Clin Oncol 27 (1): 106-13, 2009.
- Le Gouill S, Milpied N, Buzyn A, et al.: Graft-versus-lymphoma effect for aggressive T-cell lymphomas in adults: a study by the Société Francaise de Greffe de Moëlle et de Thérapie Cellulaire. J Clin Oncol 26 (14): 2264-71, 2008.
- d'Amore F, Relander T, Lauritzsen GF, et al.: Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J Clin Oncol 30 (25): 3093-9, 2012.
- Schmitz N, Lenz G, Stelljes M: Allogeneic hematopoietic stem cell transplantation for T-cell lymphomas. Blood 132 (3): 245-253, 2018.
- Park SI, Horwitz SM, Foss FM, et al.: The role of autologous stem cell transplantation in patients with nodal peripheral T-cell lymphomas in first complete remission: Report from COMPLETE, a prospective, multicenter cohort study. Cancer 125 (9): 1507-1517, 2019.
- Brink M, Meeuwes FO, van der Poel MWM, et al.: Impact of etoposide and ASCT on survival among patients aged <65 years with stage II to IV PTCL: a population-based cohort study. Blood 140 (9): 1009-1019, 2022.
- Los-de Vries GT, de Boer M, van Dijk E, et al.: Chromosome 20 loss is characteristic of breast implant-associated anaplastic large cell lymphoma. Blood 136 (25): 2927-2932, 2020.
- Schmitz N, Truemper L, Bouabdallah K, et al.: A randomized phase 3 trial of autologous vs allogeneic transplantation as part of first-line therapy in poor-risk peripheral T-NHL. Blood 137 (19): 2646-2656, 2021.
- O'Connor OA, Pro B, Pinter-Brown L, et al.: Pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma: results from the pivotal PROPEL study. J Clin Oncol 29 (9): 1182-9, 2011.
- Coiffier B, Pro B, Prince HM, et al.: Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J Clin Oncol 30 (6): 631-6, 2012.
- Amengual JE, Lichtenstein R, Lue J, et al.: A phase 1 study of romidepsin and pralatrexate reveals marked activity in relapsed and refractory T-cell lymphoma. Blood 131 (4): 397-407, 2018.
- Damaj G, Gressin R, Bouabdallah K, et al.: Results from a prospective, open-label, phase II trial of bendamustine in refractory or relapsed T-cell lymphomas: the BENTLY trial. J Clin Oncol 31 (1): 104-10, 2013.
- O'Connor OA, Horwitz S, Masszi T, et al.: Belinostat in Patients With Relapsed or Refractory Peripheral T-Cell Lymphoma: Results of the Pivotal Phase II BELIEF (CLN-19) Study. J Clin Oncol 33 (23): 2492-9, 2015.
- Fanale MA, Horwitz SM, Forero-Torres A, et al.: Five-year outcomes for frontline brentuximab vedotin with CHP for CD30-expressing peripheral T-cell lymphomas. Blood 131 (19): 2120-2124, 2018.
Treatment of Extranodal Natural Killer / T-Cell Lymphoma
Extranodal natural killer (NK)/T-cell lymphoma (nasal type) is an aggressive lymphoma marked by extensive necrosis and angioinvasion, most often presenting in extranodal sites, in particular the nasal or paranasal sinus region.[1] Other extranodal sites include the palate, trachea, skin, and gastrointestinal tract. Hemophagocytic syndrome may occur; historically, these tumors were considered part of lethal midline granuloma.[2] In most cases, Epstein-Barr virus (EBV) genomes are detectable in the tumor cells and immunophenotyping shows CD56 positivity. Cases with blood and marrow involvement are considered NK-cell leukemia. A benign NK-cell enteropathy (EBV negative) on endoscopic biopsy can be distinguished from NK/T-cell lymphoma.[3]
The increased risk of central nervous system involvement and of local recurrence has led to recommendations for local radiation therapy given before the start of chemotherapy or between cycle two and three of chemotherapy, and for intrathecal prophylaxis and/or prophylactic cranial radiation therapy.[4,5,6,7,8,9,10,11]
- A retrospective review included 1,273 patients with early-stage disease. Patients were stratified into a low-risk group and high-risk group using stage, age, lactate dehydrogenase level, performance status, and primary tumor invasion.
- A retrospective review included 303 previously untreated patients from an international consortium who received nonanthracycline chemotherapy.[15]
- The overall survival rates were identical (72%−74% at 5 years) for patients with early-stage disease who received either concurrent chemotherapy and radiation therapy or chemotherapy followed by radiation therapy.[15][Level of evidence C3]
Higher doses of radiation therapy administered at more than 50 Gy are associated with improved outcomes according to anecdotal reports.[10] The highly aggressive course, with poor response and short survival with standard therapies, especially for patients with advanced-stage disease or extranasal presentation, has led some investigators to recommend autologous or allogeneic peripheral stem cell transplant consolidation.[11,16,17,18,19,20][Level of evidence C3] Asparaginase-containing regimens have shown anecdotal response rates greater than 50% for patients with relapsing, refractory, or newly diagnosed disease.[11,21,22,23,24][Level of evidence C3] Because of the lack of randomized clinical trials with more than 100 patients for this rare type of T-cell lymphoma, regimens containing pegaspargase have become the standard for systemic therapy. Pegaspargase is a less toxic formulation of asparaginase with less hypersensitivity reactions and a longer half-life.[25,26] NK/T-cell lymphoma that presents only in the skin has a more favorable prognosis, especially in patients with coexpression of CD30 with CD56.[27]
- In a phase II trial, the anti-programmed death-ligand 1 (PD-L1) antibody avelumab was given to 21 patients with relapsed or refractory disease.[28]
- The complete response rate was 24% and the overall response rate was 38%. The responses correlated with tumor PD-L1 expression.[28][Level of evidence C3]
Treatment with pembrolizumab, an anti-programmed cell death protein 1 (PD-1) antibody, resulted in similar responses in patients with relapsed or refractory disease.[29][Level of evidence C3]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References:
- Tse E, Kwong YL: How I treat NK/T-cell lymphomas. Blood 121 (25): 4997-5005, 2013.
- Rizvi MA, Evens AM, Tallman MS, et al.: T-cell non-Hodgkin lymphoma. Blood 107 (4): 1255-64, 2006.
- Mansoor A, Pittaluga S, Beck PL, et al.: NK-cell enteropathy: a benign NK-cell lymphoproliferative disease mimicking intestinal lymphoma: clinicopathologic features and follow-up in a unique case series. Blood 117 (5): 1447-52, 2011.
- Li YX, Yao B, Jin J, et al.: Radiotherapy as primary treatment for stage IE and IIE nasal natural killer/T-cell lymphoma. J Clin Oncol 24 (1): 181-9, 2006.
- Lee J, Suh C, Park YH, et al.: Extranodal natural killer T-cell lymphoma, nasal-type: a prognostic model from a retrospective multicenter study. J Clin Oncol 24 (4): 612-8, 2006.
- Li CC, Tien HF, Tang JL, et al.: Treatment outcome and pattern of failure in 77 patients with sinonasal natural killer/T-cell or T-cell lymphoma. Cancer 100 (2): 366-75, 2004.
- Yamaguchi M, Tobinai K, Oguchi M, et al.: Phase I/II study of concurrent chemoradiotherapy for localized nasal natural killer/T-cell lymphoma: Japan Clinical Oncology Group Study JCOG0211. J Clin Oncol 27 (33): 5594-600, 2009.
- Kim SJ, Kim K, Kim BS, et al.: Phase II trial of concurrent radiation and weekly cisplatin followed by VIPD chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal NK/T-Cell Lymphoma: Consortium for Improving Survival of Lymphoma study. J Clin Oncol 27 (35): 6027-32, 2009.
- Li YX, Fang H, Liu QF, et al.: Clinical features and treatment outcome of nasal-type NK/T-cell lymphoma of Waldeyer ring. Blood 112 (8): 3057-64, 2008.
- Vargo JA, Patel A, Glaser SM, et al.: The impact of the omission or inadequate dosing of radiotherapy in extranodal natural killer T-cell lymphoma, nasal type, in the United States. Cancer 123 (16): 3176-3185, 2017.
- Yamaguchi M, Suzuki R, Oguchi M: Advances in the treatment of extranodal NK/T-cell lymphoma, nasal type. Blood 131 (23): 2528-2540, 2018.
- Yang Y, Cao JZ, Lan SM, et al.: Association of Improved Locoregional Control With Prolonged Survival in Early-Stage Extranodal Nasal-Type Natural Killer/T-Cell Lymphoma. JAMA Oncol 3 (1): 83-91, 2017.
- Yang Y, Zhu Y, Cao JZ, et al.: Risk-adapted therapy for early-stage extranodal nasal-type NK/T-cell lymphoma: analysis from a multicenter study. Blood 126 (12): 1424-32; quiz 1517, 2015.
- Yamaguchi M, Suzuki R, Oguchi M, et al.: Treatments and Outcomes of Patients With Extranodal Natural Killer/T-Cell Lymphoma Diagnosed Between 2000 and 2013: A Cooperative Study in Japan. J Clin Oncol 35 (1): 32-39, 2017.
- Kwong YL, Kim SJ, Tse E, et al.: Sequential chemotherapy/radiotherapy was comparable with concurrent chemoradiotherapy for stage I/II NK/T-cell lymphoma. Ann Oncol 29 (1): 256-263, 2018.
- Liang R, Todd D, Chan TK, et al.: Treatment outcome and prognostic factors for primary nasal lymphoma. J Clin Oncol 13 (3): 666-70, 1995.
- Cheung MM, Chan JK, Lau WH, et al.: Primary non-Hodgkin's lymphoma of the nose and nasopharynx: clinical features, tumor immunophenotype, and treatment outcome in 113 patients. J Clin Oncol 16 (1): 70-7, 1998.
- Hausdorff J, Davis E, Long G, et al.: Non-Hodgkin's lymphoma of the paranasal sinuses: clinical and pathological features, and response to combined-modality therapy. Cancer J Sci Am 3 (5): 303-11, 1997 Sep-Oct.
- Le Gouill S, Milpied N, Buzyn A, et al.: Graft-versus-lymphoma effect for aggressive T-cell lymphomas in adults: a study by the Société Francaise de Greffe de Moëlle et de Thérapie Cellulaire. J Clin Oncol 26 (14): 2264-71, 2008.
- Au WY, Weisenburger DD, Intragumtornchai T, et al.: Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood 113 (17): 3931-7, 2009.
- Jaccard A, Gachard N, Marin B, et al.: Efficacy of L-asparaginase with methotrexate and dexamethasone (AspaMetDex regimen) in patients with refractory or relapsing extranodal NK/T-cell lymphoma, a phase 2 study. Blood 117 (6): 1834-9, 2011.
- Yamaguchi M, Kwong YL, Kim WS, et al.: Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: the NK-Cell Tumor Study Group study. J Clin Oncol 29 (33): 4410-6, 2011.
- Li JW, Li YJ, Zhong MZ, et al.: Efficacy and tolerance of GELOXD/P-GEMOXD in newly diagnosed nasal-type extranodal NK/T-cell lymphoma: A multicenter retrospective study. Eur J Haematol 100 (3): 247-256, 2018.
- Wei L, Wang L, Cong J, et al.: SVILE regimen, a combination of dexamethasone, vindesine, ifosfamide, pegaspargase, and etoposide, for treating relapsed/refractory extranodal natural killer/T-cell lymphoma, nasal type. Leuk Res 96: 106422, 2020.
- Liang R, Gao GX, Chen JP, et al.: A phase 2 study of methotrexate, etoposide, dexamethasone, and pegaspargase chemotherapy for newly diagnosed, relapsed, or refractory extranodal natural killer/T-cell lymphoma, nasal type: a multicenter trial in Northwest China. Hematol Oncol 35 (4): 619-629, 2017.
- Wang X, Zhang L, Liu X, et al.: Efficacy and Safety of a Pegasparaginase-Based Chemotherapy Regimen vs an L-asparaginase-Based Chemotherapy Regimen for Newly Diagnosed Advanced Extranodal Natural Killer/T-Cell Lymphoma: A Randomized Clinical Trial. JAMA Oncol 8 (7): 1035-1041, 2022.
- Mraz-Gernhard S, Natkunam Y, Hoppe RT, et al.: Natural killer/natural killer-like T-cell lymphoma, CD56+, presenting in the skin: an increasingly recognized entity with an aggressive course. J Clin Oncol 19 (8): 2179-88, 2001.
- Kim SJ, Lim JQ, Laurensia Y, et al.: Avelumab for the treatment of relapsed or refractory extranodal NK/T-cell lymphoma: an open-label phase 2 study. Blood 136 (24): 2754-2763, 2020.
- Kwong YL, Chan TSY, Tan D, et al.: PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood 129 (17): 2437-2442, 2017.
Treatment of Enteropathy-Type Intestinal T-Cell Lymphoma
Enteropathy-type intestinal T-cell lymphoma involves the small bowel of patients with gluten-sensitive enteropathy (celiac sprue).[1,2,3,4] Because a gluten-free diet prevents the development of lymphoma in patients with celiac disease, patients diagnosed in childhood rarely develop lymphoma. The diagnosis of celiac disease is usually made by finding villous atrophy in the resected intestine. Surgery is often required for diagnosis and to avoid perforation during therapy.
Therapy is with doxorubicin-based combination chemotherapy, but relapse rates appear higher than for comparably staged diffuse large cell lymphoma.[2,4,5] Complications of treatment include gastrointestinal bleeding, small bowel perforation, and enterocolic fistulae; patients often require parenteral nutrition. For more information on parenteral nutrition, see Nutrition in Cancer Care. Multifocal intestinal perforations and visceral abdominal involvement are seen at the time of relapse. High-dose therapy with hematopoietic stem cell rescue has been used at first remission or at relapse.[2,6,7][Level of evidence C3] Evidence for this approach is anecdotal.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References:
- Egan LJ, Walsh SV, Stevens FM, et al.: Celiac-associated lymphoma. A single institution experience of 30 cases in the combination chemotherapy era. J Clin Gastroenterol 21 (2): 123-9, 1995.
- Gale J, Simmonds PD, Mead GM, et al.: Enteropathy-type intestinal T-cell lymphoma: clinical features and treatment of 31 patients in a single center. J Clin Oncol 18 (4): 795-803, 2000.
- Rizvi MA, Evens AM, Tallman MS, et al.: T-cell non-Hodgkin lymphoma. Blood 107 (4): 1255-64, 2006.
- Di Sabatino A, Biagi F, Gobbi PG, et al.: How I treat enteropathy-associated T-cell lymphoma. Blood 119 (11): 2458-68, 2012.
- Daum S, Ullrich R, Heise W, et al.: Intestinal non-Hodgkin's lymphoma: a multicenter prospective clinical study from the German Study Group on Intestinal non-Hodgkin's Lymphoma. J Clin Oncol 21 (14): 2740-6, 2003.
- Le Gouill S, Milpied N, Buzyn A, et al.: Graft-versus-lymphoma effect for aggressive T-cell lymphomas in adults: a study by the Société Francaise de Greffe de Moëlle et de Thérapie Cellulaire. J Clin Oncol 26 (14): 2264-71, 2008.
- Sieniawski M, Angamuthu N, Boyd K, et al.: Evaluation of enteropathy-associated T-cell lymphoma comparing standard therapies with a novel regimen including autologous stem cell transplantation. Blood 115 (18): 3664-70, 2010.
Treatment of Hepatosplenic T-Cell Lymphoma
Hepatosplenic T-cell lymphoma (HSTCL) is an unusual type of peripheral T-cell lymphoma occurring mostly in young men. HSTCL appears to be localized to the hepatic and splenic sinusoids, with cell surface expression of the T-cell receptor gamma/delta.[1,2,3] This lymphoma has an extremely poor prognosis and an extremely aggressive clinical course. HSTCL is treated with induction chemotherapy and stem cell transplant (SCT) consolidation.[3,4]
- A meta-analysis of 118 patients with HSTCL compared CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) and CHOP-like induction regimens.[5]
- Non–CHOP-based regimens (containing cytarabine, etoposide, and/or platinum-based treatment) were associated with improved outcomes, including an overall response rate of 82% versus 52% (P = .006) and a median overall survival (OS) of 36.5 months versus 18 months (P = .00014).
- Consolidation with allogeneic SCT was associated with an improved median OS of 33 months, versus 27 months (P = .016) for autologous SCT.[5][Level of evidence C3]
The use of ICE (ifosfamide, carboplatin, and etoposide) or IVAC (ifosfamide, etoposide, and high-dose cytarabine) has resulted in improved responses when compared with CHOP in other smaller studies as well.[6][Level of evidence D] Given the inadequate responses to CHOP or CHOP-like regimens, many clinicians are using ICE chemotherapy as front-line induction therapy, followed by consolidative allogeneic SCT for patients achieving a first remission. However, the efficacy of this approach is undetermined.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References:
- Belhadj K, Reyes F, Farcet JP, et al.: Hepatosplenic gammadelta T-cell lymphoma is a rare clinicopathologic entity with poor outcome: report on a series of 21 patients. Blood 102 (13): 4261-9, 2003.
- Chanan-Khan A, Islam T, Alam A, et al.: Long-term survival with allogeneic stem cell transplant and donor lymphocyte infusion following salvage therapy with anti-CD52 monoclonal antibody (Campath) in a patient with alpha/beta hepatosplenic T-cell non-Hodgkin's lymphoma. Leuk Lymphoma 45 (8): 1673-5, 2004.
- Pro B, Allen P, Behdad A: Hepatosplenic T-cell lymphoma: a rare but challenging entity. Blood 136 (18): 2018-2026, 2020.
- Le Gouill S, Milpied N, Buzyn A, et al.: Graft-versus-lymphoma effect for aggressive T-cell lymphomas in adults: a study by the Société Francaise de Greffe de Moëlle et de Thérapie Cellulaire. J Clin Oncol 26 (14): 2264-71, 2008.
- Klebaner D, Koura D, Tzachanis D, et al.: Intensive Induction Therapy Compared With CHOP for Hepatosplenic T-cell Lymphoma. Clin Lymphoma Myeloma Leuk 20 (7): 431-437.e2, 2020.
- Voss MH, Lunning MA, Maragulia JC, et al.: Intensive induction chemotherapy followed by early high-dose therapy and hematopoietic stem cell transplantation results in improved outcome for patients with hepatosplenic T-cell lymphoma: a single institution experience. Clin Lymphoma Myeloma Leuk 13 (1): 8-14, 2013.
Treatment of Adult T-Cell Leukemia / Lymphoma
Adult T-cell leukemia/lymphoma (ATLL) is caused by infection with the retrovirus human T-lymphotrophic virus 1 and is frequently associated with lymphadenopathy, hypercalcemia, circulating leukemic cells, bone and skin involvement, hepatosplenomegaly, a rapidly progressive course, and poor response to combination chemotherapy.[1,2] ATLL has been divided into four clinical subtypes:[3,4]
- Acute (aggressive course with leukemia, with or without extranodal or nodal involvement).
- Lymphoma (aggressive course with lymphadenopathy and no leukemia).
- Chronic (indolent course with leukemia and lymphadenopathy).
- Smoldering (indolent course with only leukemia).
The acute and lymphoma types of ATLL respond poorly to combination chemotherapy and allogeneic stem cell transplant (SCT), with a median overall survival (OS) under 1 year.[5,6,7] Less than 10% of 807 patients who received combination therapy were alive after 4 years.[7] Anecdotal durable remissions have been reported after allogeneic SCT and even after subsequent donor lymphocyte infusion for relapses after transplant.[8][Level of evidence C3] Among 815 patients who underwent allogeneic SCT in two retrospective reviews, the 3-year OS rates were 36% and 26%.[9,10][Level of evidence C1]
The combination of zidovudine and interferon-alpha has activity against ATLL, even for patients who failed previous cytotoxic therapy. Durable remissions are seen in most patients treated with this combination, but are not seen in patients with the lymphoma subtype of ATLL.[11,12,13,14,15] In a multicenter phase II study of 26 relapsed patients, 42% responded to lenalidomide (including four complete responses).[16][Level of evidence C3] Symptomatic local progression of all subtypes responds well to palliative radiation therapy.[17] In the relapsed setting, an overall response rate above 50% was seen using mogamulizumab, a humanized monoclonal antibody against the C-C chemokine receptor 4 (CCR4).[18][Level of evidence C3] For CD30-positive cases, brentuximab vedotin combined with cyclophosphamide, doxorubicin, and prednisone is the standard of care.[19] Patients with ATLL were included in the clinical trial involving mostly patients with anaplastic large cell lymphoma; a benefit for this smaller ATLL subgroup cannot be established.[19][Level of evidence C3] For more information, see the Treatment of Anaplastic Large Cell Lymphoma section.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References:
- Höllsberg P, Hafler DA: Seminars in medicine of the Beth Israel Hospital, Boston. Pathogenesis of diseases induced by human lymphotropic virus type I infection. N Engl J Med 328 (16): 1173-82, 1993.
- Foss FM, Aquino SL, Ferry JA: Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 10-2003. A 72-year-old man with rapidly progressive leukemia, rash, and multiorgan failure. N Engl J Med 348 (13): 1267-75, 2003.
- Shimoyama M: Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984-87). Br J Haematol 79 (3): 428-37, 1991.
- Takasaki Y, Iwanaga M, Imaizumi Y, et al.: Long-term study of indolent adult T-cell leukemia-lymphoma. Blood 115 (22): 4337-43, 2010.
- Yamada Y, Tomonaga M, Fukuda H, et al.: A new G-CSF-supported combination chemotherapy, LSG15, for adult T-cell leukaemia-lymphoma: Japan Clinical Oncology Group Study 9303. Br J Haematol 113 (2): 375-82, 2001.
- Fukushima T, Miyazaki Y, Honda S, et al.: Allogeneic hematopoietic stem cell transplantation provides sustained long-term survival for patients with adult T-cell leukemia/lymphoma. Leukemia 19 (5): 829-34, 2005.
- Katsuya H, Yamanaka T, Ishitsuka K, et al.: Prognostic index for acute- and lymphoma-type adult T-cell leukemia/lymphoma. J Clin Oncol 30 (14): 1635-40, 2012.
- Itonaga H, Tsushima H, Taguchi J, et al.: Treatment of relapsed adult T-cell leukemia/lymphoma after allogeneic hematopoietic stem cell transplantation: the Nagasaki Transplant Group experience. Blood 121 (1): 219-25, 2013.
- Ishida T, Hishizawa M, Kato K, et al.: Allogeneic hematopoietic stem cell transplantation for adult T-cell leukemia-lymphoma with special emphasis on preconditioning regimen: a nationwide retrospective study. Blood 120 (8): 1734-41, 2012.
- Katsuya H, Ishitsuka K, Utsunomiya A, et al.: Treatment and survival among 1594 patients with ATL. Blood 126 (24): 2570-7, 2015.
- Gill PS, Harrington W, Kaplan MH, et al.: Treatment of adult T-cell leukemia-lymphoma with a combination of interferon alfa and zidovudine. N Engl J Med 332 (26): 1744-8, 1995.
- Matutes E, Taylor GP, Cavenagh J, et al.: Interferon alpha and zidovudine therapy in adult T-cell leukaemia lymphoma: response and outcome in 15 patients. Br J Haematol 113 (3): 779-84, 2001.
- Hermine O, Allard I, Lévy V, et al.: A prospective phase II clinical trial with the use of zidovudine and interferon-alpha in the acute and lymphoma forms of adult T-cell leukemia/lymphoma. Hematol J 3 (6): 276-82, 2002.
- Bazarbachi A, Plumelle Y, Carlos Ramos J, et al.: Meta-analysis on the use of zidovudine and interferon-alfa in adult T-cell leukemia/lymphoma showing improved survival in the leukemic subtypes. J Clin Oncol 28 (27): 4177-83, 2010.
- Bazarbachi A, Suarez F, Fields P, et al.: How I treat adult T-cell leukemia/lymphoma. Blood 118 (7): 1736-45, 2011.
- Ishida T, Fujiwara H, Nosaka K, et al.: Multicenter Phase II Study of Lenalidomide in Relapsed or Recurrent Adult T-Cell Leukemia/Lymphoma: ATLL-002. J Clin Oncol 34 (34): 4086-4093, 2016.
- Simone CB, Morris JC, Stewart DM, et al.: Radiation therapy for the management of patients with HTLV-1-associated adult T-cell leukemia/lymphoma. Blood 120 (9): 1816-9, 2012.
- Ureshino H, Kamachi K, Kimura S: Mogamulizumab for the Treatment of Adult T-cell Leukemia/Lymphoma. Clin Lymphoma Myeloma Leuk 19 (6): 326-331, 2019.
- Horwitz S, O'Connor OA, Pro B, et al.: Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet 393 (10168): 229-240, 2019.
Treatment of Relapsed or Refractory Peripheral T-Cell Lymphoma
Treatment Options for Relapsed or Refractory Peripheral T-Cell Lymphoma
Treatment options for relapsed or refractory peripheral T-cell lymphoma include the following:
- Combination chemotherapy.
- ICE (ifosfamide, carboplatin, and etoposide).[1,2]
- GEMOX (gemcitabine, oxaliplatin, and dexamethasone).[3]
- DHAP (dexamethasone, high-dose cytarabine, and cisplatin).[4]
- ESHAP (etoposide, methylprednisolone, high-dose cytarabine, and cisplatin).[5,6]
- Hyper-CVAD (cyclophosphamide, vincristine, doxorubicin, and dexamethasone).[7]
- Antibody conjugates.
- Brentuximab vedotin (for CD30-positive patients).
- Histone deacetylase inhibitors.
- Dihydrofolate reductase inhibitors.
- Pralatrexate.[11]
- Anti-CD52 monoclonal antibodies.
- Alemtuzumab (only available through a restricted distribution program).
- Stem cell transplant.[12,13,14,15,16]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References:
- Jerkeman M, Leppä S, Kvaløy S, et al.: ICE (ifosfamide, carboplatin, etoposide) as second-line chemotherapy in relapsed or primary progressive aggressive lymphoma--the Nordic Lymphoma Group experience. Eur J Haematol 73 (3): 179-82, 2004.
- Zelenetz AD, Hamlin P, Kewalramani T, et al.: Ifosfamide, carboplatin, etoposide (ICE)-based second-line chemotherapy for the management of relapsed and refractory aggressive non-Hodgkin's lymphoma. Ann Oncol 14 (Suppl 1): i5-10, 2003.
- Shen QD, Wang L, Zhu HY, et al.: Gemcitabine, oxaliplatin and dexamethasone (GemDOx) as salvage therapy for relapsed or refractory diffuse large B-cell lymphoma and peripheral T-cell lymphoma. J Cancer 12 (1): 163-169, 2021.
- Schmitz N, Truemper L, Bouabdallah K, et al.: A randomized phase 3 trial of autologous vs allogeneic transplantation as part of first-line therapy in poor-risk peripheral T-NHL. Blood 137 (19): 2646-2656, 2021.
- Velasquez WS, McLaughlin P, Tucker S, et al.: ESHAP--an effective chemotherapy regimen in refractory and relapsing lymphoma: a 4-year follow-up study. J Clin Oncol 12 (6): 1169-76, 1994.
- Mercadal S, Briones J, Xicoy B, et al.: Intensive chemotherapy (high-dose CHOP/ESHAP regimen) followed by autologous stem-cell transplantation in previously untreated patients with peripheral T-cell lymphoma. Ann Oncol 19 (5): 958-63, 2008.
- Hapgood G, Stone JM, Zannino D, et al.: A phase II study of a modified hyper-CVAD frontline therapy for patients with adverse risk diffuse large B-cell and peripheral T-cell non-Hodgkin lymphoma. Leuk Lymphoma 60 (4): 904-911, 2019.
- Iyer SP, Foss FF: Romidepsin for the Treatment of Peripheral T-Cell Lymphoma. Oncologist 20 (9): 1084-91, 2015.
- Campbell P, Thomas CM: Belinostat for the treatment of relapsed or refractory peripheral T-cell lymphoma. J Oncol Pharm Pract 23 (2): 143-147, 2017.
- O'Connor OA, Horwitz S, Masszi T, et al.: Belinostat in Patients With Relapsed or Refractory Peripheral T-Cell Lymphoma: Results of the Pivotal Phase II BELIEF (CLN-19) Study. J Clin Oncol 33 (23): 2492-9, 2015.
- O'Connor OA, Pro B, Pinter-Brown L, et al.: Pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma: results from the pivotal PROPEL study. J Clin Oncol 29 (9): 1182-9, 2011.
- Schmitz N, Lenz G, Stelljes M: Allogeneic hematopoietic stem cell transplantation for T-cell lymphomas. Blood 132 (3): 245-253, 2018.
- Chen AI, McMillan A, Negrin RS, et al.: Long-term results of autologous hematopoietic cell transplantation for peripheral T cell lymphoma: the Stanford experience. Biol Blood Marrow Transplant 14 (7): 741-7, 2008.
- Hamadani M, Ngoya M, Sureda A, et al.: Outcome of allogeneic transplantation for mature T-cell lymphomas: impact of donor source and disease characteristics. Blood Adv 6 (3): 920-930, 2022.
- Mehta-Shah N, Kommalapati A, Teja S: Successful treatment of mature T-cell lymphoma with allogeneic stem cell transplantation: the largest multicenter retrospective analysis. [Abstract] Blood 136 (Suppl 1): A-624, 35-36, 2020.
- Sterling CH, Hughes MS, Tsai HL, et al.: Allogeneic Blood or Marrow Transplantation with Post-Transplantation Cyclophosphamide for Peripheral T Cell Lymphoma: The Importance of Graft Source. Transplant Cell Ther 29 (4): 267.e1-267.e5, 2023.
Changes to This Summary (09 / 21 / 2023)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Editorial changes were made to this summary.
This summary is written and maintained by the PDQ Adult Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® Cancer Information for Health Professionals pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of adult peripheral T-cell non-Hodgkin lymphoma. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Adult Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Peripheral T-Cell Non-Hodgkin Lymphoma Treatment are:
- Eric J. Seifter, MD (Johns Hopkins University)
- Cole H. Sterling, MD (Johns Hopkins University)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Adult Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as "NCI's PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary]."
The preferred citation for this PDQ summary is:
PDQ® Adult Treatment Editorial Board. PDQ Peripheral T-Cell Non-Hodgkin Lymphoma Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/lymphoma/hp/peripheral-t-cell-lymphoma-pdq. Accessed <MM/DD/YYYY>.
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either "standard" or "under clinical evaluation." These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website's Email Us.
Last Revised: 2023-09-21
Topic Contents
- General Information About Peripheral T-Cell Non-Hodgkin Lymphoma
- Late Effects of Treatment of Peripheral T-Cell Non-Hodgkin Lymphoma
- Cellular Classification of Peripheral T-Cell Non-Hodgkin Lymphoma
- Stage Information for Peripheral T-Cell Non-Hodgkin Lymphoma
- Treatment of Anaplastic Large Cell Lymphoma
- Treatment of Angioimmunoblastic T-Cell Lymphoma
- Treatment of Peripheral T-Cell Lymphoma, Not Otherwise Specified
- Treatment of Extranodal Natural Killer / T-Cell Lymphoma
- Treatment of Enteropathy-Type Intestinal T-Cell Lymphoma
- Treatment of Hepatosplenic T-Cell Lymphoma
- Treatment of Adult T-Cell Leukemia / Lymphoma
- Treatment of Relapsed or Refractory Peripheral T-Cell Lymphoma
- Changes to This Summary (09 / 21 / 2023)
- About This PDQ Summary
This information does not replace the advice of a doctor. Healthwise, Incorporated, disclaims any warranty or liability for your use of this information. Your use of this information means that you agree to the Terms of Use. Learn how we develop our content.